Lead Acid Battery
|
Lead-acid batteries, invented in 1859 by French physicist Gaston Planté, are the oldest type of rechargeable battery. Despite having a very low energy-to-weight ratio and a low energy-to-volume ratio, their ability to supply high surge currents means that the cells maintain a relatively large power-to-weight ratio. These features, along with their low cost, make them attractive for use in motor vehicles to provide the high current required by automobile starter motors. ElectrochemistryIn the charged state, each cell contains electrodes of elemental lead (Pb) and Lead(IV) Oxide (PbO2) in an electrolyte of approximately 33.5% v/v (4.2 Molar) sulfuric acid (H2SO4). In the discharged state both electrodes turn into lead(II) sulfate (PbSO4) and the electrolyte loses its dissolved sulfuric acid and becomes primarily water. Due to the freezing-point depression of water, as the battery discharges and the concentration of sulfuric acid decreases, the electrolyte is more likely to freeze during winter weather. The chemical reactions are (discharged to charged): Anode
(oxidation): Cathode (reduction): Because of the open cells with liquid electrolyte in most lead-acid batteries, overcharging with high charging voltages generates oxygen and hydrogen gas by electrolysis of water, forming an explosive mix. The acid electrolyte is also corrosive. Practical cells are usually not made with pure lead but have small amounts of antimony, tin, calcium or selenium alloyed in the plate material to strengthen it and make simplify manufacture. Voltages for common usagesThese are general voltage ranges for six-cell lead-acid batteries:
Portable batteries, such as for miners' cap lamps (headlamps) typically have two cells, and use one third of these voltages. Measuring the charge level
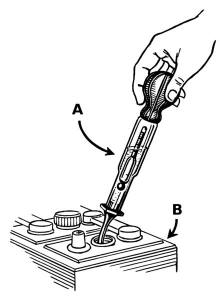
Because the electrolyte takes part in the charge-discharge reaction, this battery has one major advantage over other chemistries. It is relatively simple to determine the state of charge by merely measuring the specific gravity (S.G.) of the electrolyte, the S.G. falling as the battery discharges. Some battery designs include a simple hydrometer using colored floating balls of differing density. When used in diesel-electric submarines, the S.G. was regularly measured and written on a blackboard in the control room to indicate how much longer the boat could remain submerged. ConstructionPlatesThe lead acid cell can be demonstrated using sheet lead plates for the two electrodes. However such a construction produces only around one ampere for roughly postcard sized plates, and for only a few minutes. Gaston Planté found a way to provide a much larger effective surface area. Planté's production method remains largely unchanged and is still used in stationary applications. Faure pasted-plate construction is typical of automotive batteries. Each plate consists of a rectangular lead grid alloyed with antimony or calcium to improve the mechanical characteristics. The holes of the grid are filled with a paste of red lead and 33% dilute sulfuric acid. (Different manufacturers vary the mixture). The paste is pressed into the holes in the grid which are slightly tapered on both sides to better retain the paste. This porous paste allows the acid to react with the lead inside the plate, increasing the surface area many fold. At this stage the positive and negative plates are similar, however expanders and additives vary their internal chemistry to assist in operation. Once dry, the plates are stacked with suitable separators and inserted in the battery container. An odd number of plates is usually used, with one more positive plate than negative. Each alternate plate is connected. The positive and negative plates are manufactured already formed, so that it is only necessary to add the sulfuric acid and the battery is ready for use. The positive plates are the chocolate brown color of Lead(IV) Oxide, and the negative are the slate gray of 'spongy' lead and in this charged state are called 'formed'. One of the problems with the plates is that the plates increase in size as the active material absorbs sulfate from the acid during discharge, and decrease as they give up the sulfate during charging. This causes the plates to gradually shed the paste. It is important that there is room underneath the plates to catch this shed material. If it reaches the plates, the cell short-circuits. The paste contains carbon black, blanc fixe (barium sulfate) and lignosulfonate. The blanc fixe acts as a seed crystal for the lead–to–lead sulfate reaction. The blanc fixe must be fully dispersed in the paste in order for it to be effective. The lignosulfonate prevents the negative plate from forming a solid mass during the discharge cycle, instead enabling the formation of long needle–like crystals. The long crystals have more surface area and are easily converted back to the original state on charging. Carbon black counteracts the effect of inhibiting formation caused by the lignosulfonates. Sulfonated naphthalene condensate dispersant is a more effective expander than lignosulfonate and speeds up formation. This dispersant improves dispersion of barium sulfate in the paste, reduces hydroset time, produces a more breakage-resistant plate, reduces fine lead particles and thereby improves handling and pasting characteristics. It extends batter life by increasing end–of–charge voltage. Sulfonated naphthalene requires about one-third to one-half the amount of lignosulfonate and is stable to higher temperatures. About 60% of the weight of an automotive-type lead-acid battery rated around 60 Ah (8.7 kg of a 14.5 kg battery) is lead or internal parts made of lead; the balance is electrolyte, separators, and the case. SeparatorsSeparators between the positive and negative plates prevent short-circuit through physical contact, mostly through dendrites (‘treeing’), but also through shedding of the active material. Separators obstruct the flow of ions between the plates and increase the internal resistance of the cell. Wood, rubber, glass fiber mat, cellulose, and PVC or polyethylene plastic have been used to make separators. Wood was the original choice, but deteriorated in the acid electrolyte. Rubber separators were stable in the battery acid. An effective separator must possess a number of mechanical properties; such as permeability, porosity, pore size distribution, specific surface area, mechanical design and strength, electrical resistance, ionic conductivity, and chemical compatibility with the electrolyte. In service, the separator must have good resistance to acid and oxidation. The area of the separator must be a little larger than the area of the plates to prevent material shorting between the plates. The separators must remain stable over the battery's operating temperature range. ApplicationsWet cell stand-by (stationary) batteries designed for deep discharge are commonly used in large backup power supplies for telephone and computer centers, grid energy storage, and off-grid household electric power systems. Lead-acid batteries are used in emergency lighting in case of power failure. Traction (propulsion) batteries are used for in golf carts and other battery electric vehicles. Large lead-acid batteries are also used to power the electric motors in diesel-electric (conventional) submarines and are used on nuclear submarines as well. Motor vehicle starting, lighting and ignition (SLI) batteries (car batteries) provides current for starting internal combustion engines. Valve-regulated lead acid batteries cannot spill their electrolyte. They are used in back-up power supplies for alarm and smaller computer systems (particularly in uninterruptible power supplies) and for electric scooters, electrified bicycles, marine applications, battery electric vehicles or micro hybrid vehicles, and motorcycles. Lead-acid batteries were used to supply the filament (heater) voltage (usually between 2 and 12 volts with 2 V being most common) in early vacuum tube (valve) radio receivers. CyclesStarting batteriesLead acid batteries designed for starting automotive engines are not designed for deep discharge. They have a large number of thin plates designed for maximum surface area, and therefore maximum current output, but which can easily be damaged by deep discharge. Repeated deep discharges will result in capacity loss and ultimately in premature failure, as the electrodes disintegrate due to mechanical stresses that arise from cycling. Starting batteries kept on continous float charge will have corrosion in the electrodes and result in premature failure. Starting batteries should be kept open circuit but charged regularly (at least once every two weeks) to prevent sulfation. Deep cycle batteriesSpecially designed deep-cycle cells are much less susceptible to degradation due to cycling, and are required for applications where the batteries are regularly discharged, such as photovoltaic systems, electric vehicles (forklift, golf cart, electric cars and other) and uninterruptible power supplies. These batteries have thicker plates that can deliver less peak current, but can withstand frequent discharging. Marine/Motorhome batteries, sometimes called "leisure batteries", are something of a compromise between the two, able to be discharged to a greater degree than automotive batteries, but less so than deep cycle batteries. Fast and slow charge and dischargeThe capacity of a lead-acid battery is not a fixed quantity but varies according to how quickly it is discharged. An empirical relationship exists between discharge rate and capacity, known as Peukert's law. When a battery is charged or discharged, this initially affects only the reacting chemicals, which are at the interface between the electrodes and the electrolyte. With time, these chemicals at the interface, which we will call an "interface charge", spread by diffusion throughout the volume of the active material. If a battery has been completely discharged (e.g. the car lights were left on overnight) and next is given a fast charge for only a few minutes, then during the short charging time it develops only a charge near the interface. After a few hours this interface charge will spread to the volume of the electrode and electrolyte, leading to an interface charge so low that it may be insufficient to start the car. On the other hand, if the battery is given a slow charge, which takes longer, then the battery will become more fully charged, since then the interface charge has time to redistribute to the volume of the electrodes and electrolyte, and yet is replenished by the charger. Similarly, if a battery is subject to a fast discharge (such as starting a car, which is a draw of some 200 amps) for a few minutes, it will appear to go dead. Most likely it has only lost its interface charge; after a wait of a few minutes it should appear to be operative. On the other hand, if a battery is subject to a slow discharge (such as leaving the car lights on, which is a draw of only 6 amps), then when the battery appears to be dead it likely has been completely discharged. Valve regulatedIn a valve regulated lead acid (VRLA) battery the hydrogen and oxygen produced in the cells largely recombine into water. Leakage is minimal, although some electrolyte still escapes if the recombination cannot keep up with gas evolution. Since VRLA batteries do not require (and make impossible) regular checking of the electrolyte level, they have been called maintenance free batteries. However, this is somewhat of a misnomer. VRLA cells do require maintenance. As electrolyte is lost, VRLA cells "dry-out" and lose capacity. This can be detected by taking regular internal resistance, conductance or impedance measurements. Regular testing reveals whether more involved testing and maintenance is required. Recent maintenance procedures have been developed allowing "rehydration", often restoring significant amounts of lost capacity. VRLA types became popular on motorcycles around 1983, because the acid electrolyte is absorbed into the separator, so it cannot spill. The separator also helps them better withstand vibration. They are also popular in stationary applications such as telecommunications sites, due to their small footprint and installation flexibility. The electrical characteristics of VRLA batteries differ somewhat from wet-cell lead-acid batteries, requiring caution in charging and discharging. SulfationLead-acid batteries lose the ability to hold a charge when discharged for too long due to sulfation, the crystallization of lead sulfate. They generate electricity through a double sulfate chemical reaction. Lead and Lead(IV) Oxide, which are the active materials on the battery's plates, react with sulfuric acid in the electrolyte to form lead sulfate. The lead sulfate first forms in a finely divided, amorphous state, and easily reverts to lead, lead oxide and sulfuric acid when the battery recharges. As batteries cycle through numerous discharge and charges, the lead sulfate slowly converts to a stable crystalline form that no longer dissolves on recharging. Thus, not all the lead is returned to the battery plates, and the amount of usable active material necessary for electricity generation declines over time. Sulfation occurs in all lead-acid batteries during normal operation. It clogs the grids, impedes recharging and ultimately expands, cracking the plates and destroying the battery. In addition, the sulfate portion (of the lead sulfate) is not returned to the electrolyte as sulfuric acid. The large crystals physically block the electrolyte from entering the pores of the plates. Sulfation can be avoided if the battery is fully recharged immediately after a discharge cycle. Sulfation also affects the charging cycle, resulting in longer charging times, less efficient and incomplete charging, and higher battery temperatures. The process can often be at least partially prevented and/or reversed by a desulfation technique called pulse conditioning, in which short but powerful current surges are repeatedly sent through the damaged battery. Over time, this procedure tends to break down and dissolve the sulfate crystals, restoring some capacity. Higher temperature speeds both desulfation and sulfation, although too much heat damages the battery by accelerating corrosion. Risk of explosionExcessive charging electrolyzes some of the water emitting hydrogen and oxygen. This process is known as "gassing". Wet cells have open vents to release any gas produced, and VRLA batteries rely on valves fitted to each cell. Wet cells come with catalytic caps to recombine any emitted hydrogen. A VRLA cell normally recombines any hydrogen and oxygen produced inside the cell, but malfunction or overheating may cause gas to build up. If this happens (e.g., by overcharging) the valve vents the gas and normalizes the pressure, producing a characteristic acid smell. Valves can sometimes fail however, if dirt and debris accumulate, allowing pressure to build up. If the accumulated hydrogen and oxygen within either a VRLA or wet cell is ignited, an explosion results. The force can burst the plastic casing or blow the top off the battery, spraying acid and casing shrapnel. An explosion in one cell may ignite the combustible gas mixture in remaining cells. The cell walls of VRLA batteries typically swell when the internal pressure rises. The deformation varies from cell to cell, and is greater at the ends where the walls are unsupported by other cells. Such over-pressurized batteries should be carefully isolated and discarded, protected by goggles, overalls, gloves, etc. Environmental concernsAccording to a 2003 report entitled, "Getting the Lead Out," by Environmental Defense and the Ecology Center of Ann Arbor, Mich., the batteries of vehicles on the road contained an estimated 2,600,000 metric tons (2,560,000 LT; 2,870,000 ST) of lead. Lead is extremely toxic. Long-term exposure to even tiny amounts of lead can cause brain and kidney damage, hearing impairment, and learning problems in children. The auto industry uses over 1,000,000 metric tons (980,000 LT; 1,100,000 ST) every year, with 90% going to conventional lead-acid vehicle batteries. While lead recycling is a well-established industry, more than 40,000 metric tons (39,000 LT; 44,000 ST) ends up in landfills every year. According to the federal Toxic Release Inventory, another 70,000 metric tons (69,000 LT; 77,000 ST) are released in the lead mining and manufacturing process. Attempts are being made to develop alternatives (particularly for automotive use) because of concerns about the environmental consequences of improper disposal and of lead smelting operations, among other reasons. Alternatives are unlikely to displace them for applications such as engine starting or backup power systems, as there is no cheaper alternative when weight is not a factor. Lead-acid battery recycling is one of the most successful recycling programs in the world. In the United States 97% of all battery lead was recycled between 1997 and 2001. An effective pollution control system is a necessity to prevent lead emission. Continuous improvement in battery recycling plants and furnace designs is required to keep pace with emission standards for lead smelters. AdditivesSince the 1950’s chemical additives have been used to reduce lead sulfate build up on plates and improve battery condition when added to the electrolyte of a vented lead-acid battery. Such treatments are rarely, if ever, effective. Two compounds used for such purposes are Epsom salts and EDTA. Epsom salts reduces the internal resistance in a weak or damaged battery and may allow a small amount of extended life. EDTA can be used to dissolve the sulfate deposits of heavily discharged plates. However, the dissolved material is then no longer available to participate in the normal charge/discharge cycle, so a battery temporarily revived with EDTA should not be expected to have normal life expectancy. Residual EDTA in the lead-acid cell forms organic acids which will accelerate corrosion of the lead plates and internal connectors. Active material changes physical form during discharge, resulting in plate growth, distortion of the active material, and shedding of active material. Once the active material has fallen out of the plates, it cannot be restored into position by any chemical treatment. Similarly, internal physical problems such as cracked plates, corroded connectors, or damaged separators cannot be restored chemically. Corrosion ProblemsCorrosion of the external metal parts of the lead-acid battery results from a chemical reaction of the battery terminals, lugs and connectors. It can be caused by the following: The corrosion on the positive terminal is caused by electrolysis, due a mismatch of metal alloys used in the manufacture of the battery terminal and cable connector. White corrosion is usually lead or zinc sulfate crystals. Aluminum connectors corrode to aluminum sulfate. Copper connectors produce blue and white corrosion crystals. Minimize corrosion by coating with a suitable rubber or plastic spray or using a commercially-available product. If the battery is over-filled with water and electrolyte, thermal expansion can force some of the liquid out of the battery vents onto the top of the battery. This solution can then react with the lead and other metals in the battery connector and cause corrosion. The electrolyte can weep from the plastic-to-lead seal where the battery terminals penetrate the plastic case. Acid fumes that vaporize through the vent caps, often caused by overcharging, and insufficient battery box ventilation can allow the sulfuric acid fumes to build up and react with the exposed metals. |