Nickel-metal Hydride Battery
|
A nickel-metal hydride cell, abbreviated NiMH, is a
type
Common AA cells (penlight-size) NiMH batteries have nominal charge capacities (C) ranging from 1100 mA·h to 2900 mA·h at 1.2 V, usually measured at a discharge rate of 0.2×C per hour. Useful discharge capacity is a decreasing function of the discharge rate, but up to a rate of around 1×C (full discharge in one hour), it does not differ significantly from the nominal capacity. The specific energy density for NiMH material is approximately 70 W·h/kg (250 kJ/kg), compared to 40–60 W·h/kg for the more common nickel-cadmium, or 100-160 W·h/kg for Li-ion. NiMH has a volumetric energy density of about 300 W·h/L (1080 MJ/m³), significantly better than nickel-cadmium at 50–150 Wh/L, and about the same as Li-ion at 250-360 W·h/L. HistoryThe first consumer grade NiMH cells for smaller applications appeared on the market in 1989 as a variation of the 1970s' Nickel hydrogen battery. Positive electrode development was done by Dr. Masahiko Oshitani from GS Yuasa Company, who was the first to develop high-energy paste electrode technology. The association of this high-energy electrode with high-energy hybrid alloys for the negative electrode, discovered by Philips Laboratories and French CNRS labs in the 1970s, led to the new environmentally-friendly high-energy NiMH cell. Battelle-Geneva's pioneering work on NiMH, 1967The pioneering work on NiMH batteries – essentially based on sintered Ti2Ni+TiNi+x alloys for the negative electrode and NiOOH-electrodes for the positives – was performed at the Battelle Geneva Research Center starting after its invention in 1967: The development work was sponsored over nearly 2 decades by Daimler-Benz Comp./Stuttgart and by Volkswagen AG. within the framework of Deutsche Automobilgesellschaft. The batteries showed high energy density up to 50 W·h/kg (180 kJ/kg), power density up to 1000 W/kg and a reasonable deep cycle life of 500 cycles (DOD=100%). Patent applications were filed in European countries (priority: Switzerland) , USA and Japan and the patents transferred to Daimler-Benz Comp./Stuttgart. Ref: Elektrode zur Speicherung und Aktivierung von Wasserstoff", Inventor: K.D. Beccu, Battelle-Geneva, Swiss Priority Application No. 6333/67-Bb3/CH/2 - (2.05.1967), Patent: DE 2317505 C2 (18.10.73). Negative electrode of Ti-Ni alloy hydride phases, US patent US 3,669,745 (06/13,1972), inventor: K.D. Beccu, Ph.D, Battelle-Geneva R&D Center. See also "History and other Information" in the Discussion Section. The NiMH cells developed by Philips Laboratories and French CNRS (see above) were based on rare earth metal alloys. They suffered from the instability of the alloys in alkaline electrolyte and consequently insufficient cycle life. Ovonic Battery Comp. in Michigan, USA altered and improved the Ti-Ni alloy structure and composition according to their patent and licensed NiMH batteries to over 50 companies worldwide. The "invented NiMH variation" of Ovonics consisted in special alloys with disordered alloy structure and specific multi-component alloy compositions. Unfortunately linked to their composition, calendar and cycle life of such alloys always remain very low, and all NiMH batteries manufactured at present time in the world consist in AB5 type rare earth metal alloys. Currently, more than 2 million hybrid cars worldwide are running with NiMH batteries,{ref: Avicenne Conf., Nice 2008, M.A. Fetcenko/ECD} e.g. Prius, Lexus (Toyota), Civic, Insight (Honda), Fusion (Ford) and others. Many of these batteries are manufactured by PEVE (Panasonic) and Sanyo. Acquisition by Chevron and CobasysStan Ovshinsky invented and patented the NiMH battery and founded Ovonics Battery Company in 1982. GM purchased the patent from Ovonics in 1994. By the late 1990s, NiMH batteries were being used successfully in many fully electric vehicles, such as the General Motors EV1 and Dodge Caravan Epic minivan. In October 2000, the patent was sold to Texaco and a week later Texaco was acquired by Chevron. Soon after this, the lack of availability of NiMH batteries became the deciding factor for the discontinuation of the American-Made electric vehicles. Chevron's Cobasys subsidiary will only provide these batteries to large OEM orders. The American EV manufacturers shut down their lines citing lack of battery availability as one of their chief obstacles. The Cobasys control of NiMH batteries has created a patent encumbrance of large automotive NiMH batteries. ApplicationsApplications of NiMH electric vehicle batteries includes all-electric plug-in vehicles such as the General Motors EV1, Honda EV Plus, Ford Ranger EV and Vectrix scooter. Hybrid vehicles such as the Toyota Prius, Honda Insight, Ford Escape Hybrid, Chevrolet Malibu Hybrid, and Honda Civic Hybrid also use them. NiMH technology is used extensively in rechargeable batteries for consumer electronics, and it will also be used on the Alstom Citadis low floor tram ordered for Nice, France; as well as the humanoid prototype robot ASIMO designed by Honda.
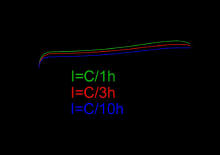
ElectrochemistryThe negative electrode reaction occurring in a NiMH cell is The charge reaction is read left-to-right and the discharge reaction is read right-to-left. On the positive electrode, nickel oxyhydroxide (NiOOH) is formed, The "metal" M in the negative electrode of a NiMH cell is actually an intermetallic compound. Many different compounds have been developed for this application, but those in current use fall into two classes. The most common is AB5, where A is a rare earth mixture of lanthanum, cerium, neodymium, praseodymium and B is nickel, cobalt, manganese, and/or aluminum. Very few cells use higher-capacity negative material electrodes based on AB2 compounds, where A is titanium and/or vanadium and B is zirconium or nickel, modified with chromium, cobalt, iron, and/or manganese, due to the reduced life performances. Any of these compounds serves the same role, reversibly forming a mixture of metal hydride compounds. When overcharged at low rates, oxygen produced at the positive electrode passes through the separator and recombines at the surface of the negative. Hydrogen evolution is suppressed and the charging energy is converted to heat. This process allows NiMH cells to remain sealed in normal operation and to be maintenance-free. NiMH cells have an alkaline electrolyte, usually potassium hydroxide. For separation hydrophilic polyolefin nonwovens are used. ChargingThe charging voltage is in the range of 1.4–1.6 V/cell. A fully-charged cell measures 1.4–1.45 V (unloaded), and supplies a nominal average 1.25 V/cell during discharge, down to about 1.0–1.1 V/cell (further discharge may cause permanent damage). This voltage varies depending on the discharge rate of the cell (lower discharge loads result in an increased voltage output for longer periods, approaching the 1.4 V unloaded cell voltage). In general, a constant-voltage charging method cannot be used for automatic charging. When fast-charging, it is advisable to charge the NiMH cells with a smart battery charger to avoid overcharging, which can damage cells and cause dangerous conditions. A NiCd charger should not be used as an automatic substitute for a NiMH charger. ΔV charging methodAccording to Panasonic and other NiMH cell manufacturers, the ΔV method is one of the preferred charging methods for charging. The charger measures the rate of change (signified by the symbol Δ) of the voltage of the cell (signified by the letter V). This is illustrated in the "NiMH charge curve" figure. The cell or battery is rapidly charged at a constant current of 1 C/h, where C is the capacity of the battery (the capacity is expressed in ampere hours, or more commonly milliampere hours (mA·h). After the cell is fully charged, and as it begins to overcharge, the voltage polarity of the electrodes inside the battery will begin to reverse, and this will cause the battery voltage to decrease slightly. A ΔV type battery charger ends the charge cycle by switching off the charging current when it senses this drop in voltage. In some cases, a very small "trickle charge" may remain. The "charge curve" graph also shows that the charge voltage will change depending on the charge current (it also changes with temperature and battery age). This generally means that a constant-voltage charging method cannot be used automatically, because it will either be unsafe, or it will not charge batteries reliably and consistently. This is unlike a lead-acid cell for example, which can, in theory, be more easily charged at a suitably chosen constant voltage. ΔT temperature charging methodThe ΔT temperature change method is similar in principle to the ΔV method. Because the charging voltage is nearly constant, if constant-current charging is used, then a near constant power is entering the cell. When the cell is charging, most of this power will be converted to chemical energy. However, when the cell is fully charged, most of the charging power will then be converted to heat. This results in an increase in the rate of change of temperature, which can be detected by a sensor measuring the battery temperature. Manual chargingIf a suitable battery charger is not available, constant-voltage or constant-current charging can be done manually, at a moderately high charging rate, if careful attention is given. For proper charging, the voltage and/or current must be set to a suitable charging rate for the particular battery, and a timer should be set. Periodic monitoring is strongly recommended to avoid overcharging (resulting in a voltage drop), or overheating (resulting in an excessive temperature rise and possibly an overpressure condition). Trickle chargingSome equipment manufacturers consider that NiMH cells can be safely charged in simple fixed, low-current chargers with or without timers, and that permanent overcharging is permissible with currents up to 0.1 C (where C is the current equivalent to the capacity of the battery divided by one hour). According to the Panasonic NiMH charging manual, extensive trickle charging can cause battery deterioration due to overcharging, and it is the least preferred charging method concerning battery performance. If it is used, the trickle charge rate should be limited to between 0.033 C and 0.05 C for a maximum of 20 hours to avoid damaging the batteries. For a slow charge, or "trickle charge" process, Duracell recommends "a maintenance charge of indefinite duration at 0.0033 C". Some chargers do this after the charge cycle, to offset the natural self-discharge rate of the battery. To maximize battery life, the preferred charge method of NiMH cells uses low duty cycle pulses of high current rather than continuous low current. SafetyA good safety feature of a custom-built charger is to use a resettable fuse in series with the cell, particularly of the bimetallic strip type. This fuse will open if either the current or the temperature goes too high. Modern NiMH cells contain catalysts to immediately deal with gases developed as a result of over-charging without being harmed (2 H2 + O2 ---catalyst → 2 H2O). However, this only works with overcharging currents of up to 0.1C (nominal capacity divided by 10 hours). As a result of this reaction, the batteries will heat up considerably, marking the end of the charging process. Some quick chargers have a fan to keep the batteries cool. A method for very rapid charging called in-cell charge control involves an internal pressure switch in the cell, which disconnects the charging current in the event of overpressure. There is an inherent risk with NiMH chemistry that overcharging will cause a buildup of hydrogen, causing the cell to rupture. Therefore, cells have a vent. Hydrogen will be emitted from the vent in the event of serious overcharging. Loss of capacityVoltage depression ("memory effect") from repeated partial discharge can occur, but is reversible through charge cycling. Patent encumbranceNiMH batteries suitable for use in electric vehicles and plug-in hybrids are tightly patented and have not been licensed for use by manufacturers, thereby slowing the development of new models. DischargingUnder a light load (0.5 ampere), the starting voltage of a freshly charged AA NiMH cell in good condition is about 1.4 volts; some measure almost 1.5 volts. This voltage falls rapidly to about 1.25 volts at 10% depth of discharge (DOD) and then remains almost constant until the cell is over 80% discharged. The voltage then falls rapidly from about 1.2 volts down to 0.8–1.0 volts at which the cell is considered "flat" in most devices. Mid-discharge at a load of 1 ampere, the output is about 1.2 volts; at 2 amperes, about 1.15 volts; the total effective differential internal resistance is about 0.05 ohms. Nickel metal hydride batteries provide a relatively constant voltage for most of the discharge cycle, unlike a standard alkaline where the voltage falls steadily during discharge. Over-dischargingA complete discharge of a cell until it goes into polarity reversal can cause permanent damage to the cell. This situation can occur in the common arrangement of four AA cells in series in a digital camera, where one will be completely discharged before the others due to small differences in capacity among the cells. When this happens, the "good" cells will start to "drive" the discharged cell in reverse, which can cause permanent damage to that cell. Some cameras, GPS receivers and PDAs detect the safe end-of-discharge voltage of the series cells and auto-shutdown, but devices like flashlights and some toys do not. A single cell driving a load won't suffer from polarity reversal, because there are no other cells to reverse-charge it when it becomes discharged. Irreversible damage from polarity reversal is a particular danger in systems, even when a low voltage threshold cutout is employed, where cells in the battery are of different temperatures. This is because the capacity of NiMH cells significantly declines as the cells are cooled. This results in a lower voltage under load of the colder cells. Self-dischargeNiMH cells historically had a somewhat higher self-discharge rate (equivalent to internal leakage) than NiCd cells. The self-discharge is 5–10% on the first day and stabilizes around 0.5–1% per day at room temperature. This is not a problem in the short term but makes them unsuitable for many light-duty uses, such as clocks, remote controls, or safety devices, where the battery would normally be expected to last many months or years. The rate is strongly affected by the temperature at which the batteries are stored with cooler storage temperatures leading to slower discharge rate and longer battery life. The highest capacity cells on the market (>8000 mA·h) are reported to have the highest self-discharge rates. Low self-discharge cellsA new type of nickel-metal hydride cell was introduced in 2005 that reduces self-discharge and therefore lengthens shelf life. By using a new separator, manufacturers claim the cells retain 70–85% of their capacity after one year when stored at 20 °C (68 °F). These cells are marketed as "hybrid", "ready-to-use" or "pre-charged" rechargeables. Besides the longer shelf life, they are otherwise similar to normal NiMH batteries of equivalent capacity and can be charged in typical NiMH chargers. Low self-discharge cells have lower capacity than some standard NiMH cells due to the larger area of the separator. The highest capacity low-self-discharge cells have 2000–2450 mA·h and 850 mA·h capacities for AA and AAA cells respectively, compared to 2800 mA·h and 1000 mA·h for standard AA and AAA cells. But C types are typically higher than their usual NiMH cousins with 4000 mA·h and the D type being 8000 mA·h. However, after only a few weeks of storage, the retained capacity of low-self-discharge batteries often exceeds that of traditional NiMH batteries of higher capacity. Environmental impactImproper disposal of NiMH batteries poses less environmental hazard than that of NiCd because of the absence of toxic cadmium. However, mining and processing the various alternate metals that form the negative electrode may be expected to pose other types of environmental impact, depending on the metal, mining method, and environmental practices of the mine. Most industrial nickel is recycled, due to the relatively easy retrieval of the metal from scrap, and due to its high value. Comparison with other battery typesNiMH cells and chargers are readily available in retail stores in the common sizes AAA and AA. Adapter sleeves are available to use the more common AA size in C and D applications. The sizes C and D cells are somewhat available, but are often just a AA core hidden in an outer shell, with a rating of about 2500 mA·h, much less than ordinary alkaline C and D batteries. Real NiMH C and D batteries are expensive (and the chargers are uncommon); they should be rated at least 5000 mA·h for C and 10,000 mA·h for D sizes. PP3 (nine volt) NiMH batteries are available; these usually have an output voltage of 8.4 V (1.2 × 7) and a capacity of roughly 200 mA·h. Also available are eight-cell nine volt batteries with a nominal output voltage of 9.6 V (1.2 × 8). NiMH cells are not expensive, and the voltage and performance is similar to primary alkaline cells in those sizes; they can be substituted for most purposes. The ability to recharge hundreds of times can save money and resources. NiMH cells are often used in digital cameras and other high drain devices, where they often vastly outperform primary batteries even on a single charge. Applications that require frequent replacement of the battery, such as toys or video game controllers, also benefit from use of rechargeable batteries. With the development of low self-discharge NiMHs (see section above), many occasional-use and very low-power applications are now candidates for NiMH cells. NiMH cells are particularly advantageous for high current drain applications, due in large part to their low internal resistance. Alkaline batteries, which might have approximately 3000 mA·h capacity at low current demand (200 mA), will have about 700 mA·h capacity with a 1000 mA load. Digital cameras with LCDs and flashlights can draw over 1000 mA, quickly depleting alkaline batteries. NiMH cells can deliver these current levels and maintain their full capacity. Certain devices that were designed to operate using primary alkaline chemistry (or zinc-carbon/chloride) cells will not function when one uses NiMH cells as substitutes. However, this is rare, as most devices compensate for the voltage drop of an alkaline as it discharges down to about 1 volt. A good-quality freshly charged NiMH cell delivers 1.4–1.45 V, very close to the 1.5 V that these devices expect. Such devices would also likely have an extremely short runtime as the voltage from an alkaline falls to 1.4 V quite quickly from the 1.5 V starting voltage. Low internal resistance allows NiMH cells to deliver a near-constant voltage until they are almost completely discharged. This will result in a battery level indicator to overstate the remaining charge if it was designed to read only the voltage curve of alkaline cells. The voltage of alkaline cells decreases steadily during most of the discharge cycle. Lithium ion batteries have a higher energy density than nickel-metal hydride batteries. But they also have a much lower shelf-life and are significantly more expensive to produce. In October 2009, ECD Ovonics announced that their next-generation NiMH batteries will provide specific energy and power that are comparable to those of lithium ion batteries at a cost that is significantly lower than the cost of lithium ion batteries. Some information extracted from Wikipedia, the free encyclopedia: Text is available under the Creative Commons Attribution-ShareAlike License; additional terms may apply. See Terms of Use for details. |