The
Baghdad Battery of antiquity

Around 1936, archaeologists uncovered in a village near Baghdad
a set of
terracotta jars which each contained a rolled-up sheet of copper
which housed an iron rod. Some scientists speculate these to be ancient
galvanic cells (roughly 2,000 years old, though the find's age is
still debated), and dubbed them the "Baghdad
Batteries". It is believed a common food acid, such as lemon juice
or vinegar, served as an electrolyte. Modern replicas have successfully
produced currents, lending credence to this hypothesis. If the
collection was indeed a battery, it could have been used for
electroplating, to produce mild electric shocks as a source of
religious experience, or simply used to store ancient
scrolls.
In a 1939 article in Astounding magazine, German rocket
scientist Willy Ley wrote:
“Dr. Wilhelm Koenig of the Iraq Museum in Bagdad reported recently
that a peculiar instrument was unearthed by an expedition of his museum
in the summer of 1936. The find was made at Khujut Rabu’a, not far to
the southeast of Bagdad. It consisted of a vase made of clay, about 14
centimeters high and with its largest diameter 8 centimeters. The
circular opening at the top of the vase had a diameter of 33
millimeters. Inside of this vase a cylinder made of sheet copper of
high purity was found—the cylinder being 10 centimeters high and having
a diameter of about 26 millimeters, almost exactly 1 inch.
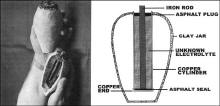
“The lower end of the copper cylinder was covered with a piece of
sheet copper, the same thickness and quality as the cylinder itself.
The inner surface of this round copper sheet—the one that formed the
inner bottom of the hollow cylinder—was covered with a layer of asphalt,
3 millimeters in thickness. A thick, heavy plug of the same material
was forced into the upper end of the cylinder. The center of the plug
was formed by a solid piece of iron—now 75 millimeters long and
originally a centimeter or so in diameter. The upper part of the iron
rod shows that it was at first round, and while the lower end has partly
corroded away so that the rod is pointed now at the lower end, it might
be safely assumed that in the beginning it was of uniform thickness.
“An assembly of this kind cannot very well have any other purpose
than that of generating a weak electric current. If one remembers that
it was found among undisturbed relics of the Parthian Kingdom—which
existed from 250 B.C. to 224 A.D.—one naturally feels very reluctant to
accept such an explanation, but there is really no alternative.
1800 - The
Voltaic Pile
In 1780,
Luigi Galvani was dissecting a frog affixed to a brass hook. When he
touched its leg with his iron scalpel, the leg twitched. Galvani
believed the energy that drove this contraction came from the leg
itself, and called it "animal electricity".
However,
Alessandro Volta, a friend and fellow scientist, disagreed,
believing this phenomenon was actually caused by two different metals
being joined together by a moist intermediary. He experimentally
verified this hypothesis, and published it in 1791. In 1800 Volta
invented the first true battery which came to be known as the
Voltaic Pile.
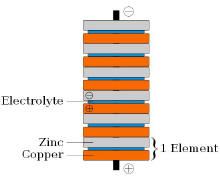
A zinc-copper Voltaic Pile.
The
Voltaic Pile consisted of pairs of copper and zinc discs piled on
top of each other, separated by a layer of cloth or cardboard soaked in
brine (i.e. the electrolyte). Unlike the
Leyden jar, the Voltaic Pile produced a continuous and stable
current, and lost little charge over time when not in use, though his
early models could not produce a voltage strong enough to produce
sparks.[2]
He experimented with various metals and found that zinc and silver gave
the best results.
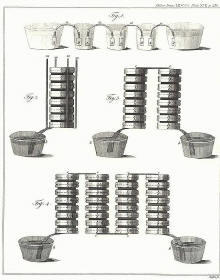
Volta's own illustrations of his Crown of Cups and Voltaic
Pile, the first batteries.
Volta believed the current was the result of two different materials
simply touching each other—an obsolete scientific theory known as
contact tension—and not the result of chemical reactions (however,
see
thermoelectric effect). Consequently, he regarded the corrosion of
the zinc plates as an unrelated flaw that could perhaps be fixed by
changing the materials somehow. However, no scientist ever succeeded in
preventing this corrosion. In fact, it was observed that the corrosion
was faster when a higher current was drawn. This suggested that the
corrosion was actually integral to the battery's ability to produce a
current. This, in part, led to the rejection of Volta's
contact tension theory in favor of electrochemical theory. Volta's
illustrations of his Crown of Cups and Voltaic Pile (first figure,
above), have extra metal disks, now know to be unnecessary, on both the
top and the bottom. The figure associated with this section, of the
zinc-copper voltaic pile, has the modern design, an indication that
"contact tension" is not the source of
electromotive force for the voltaic pile.
Volta's original pile models had some technical flaws, one of them
involving the electrolyte leaking and causing short-circuits due to the
weight of the discs compressing the brine-soaked
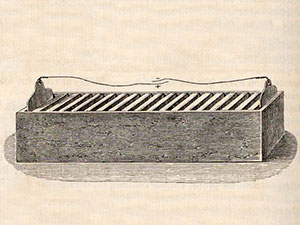 cloth. An Englishman
named
William Cruickshank solved this problem by laying the elements in a
box instead of piling them in a stack. This was known as the
trough battery.
Volta himself invented a variant which consisted of a chain of cups
filled with a salt solution, linked together by metallic arcs dipped
into the liquid. This was known as the Crown of Cups. These arcs were
made of two different metals (e.g. zinc and copper) soldered together.
This model also proved to be more efficient than his original piles,
though it didn't prove as popular.
cloth. An Englishman
named
William Cruickshank solved this problem by laying the elements in a
box instead of piling them in a stack. This was known as the
trough battery.
Volta himself invented a variant which consisted of a chain of cups
filled with a salt solution, linked together by metallic arcs dipped
into the liquid. This was known as the Crown of Cups. These arcs were
made of two different metals (e.g. zinc and copper) soldered together.
This model also proved to be more efficient than his original piles,
though it didn't prove as popular.
Another problem with Volta's batteries was short battery life (an
hour's worth at best), which was caused by two phenomena. The first was
that the current produced electrolysed the electrolyte solution,
resulting in a film of hydrogen bubbles forming on the copper, which
steadily increased the internal resistance of the battery (This effect,
called polarization, is counteracted in modern cells by
additional measures). The other was a phenomenon called local action,
wherein minute short-circuits would form around impurities in the zinc,
causing the zinc to degrade. The latter problem was solved in 1835 by
William Sturgeon, who found that mixing some
mercury into the zinc eliminated the local action.
Despite its flaws, Volta's batteries provided a steadier current than
Leyden jars, and made possible many new experiments and discoveries,
such as the first electrolysis of water by Anthony Carlisle and William
Nicholson.
1836 - The
Daniell cell
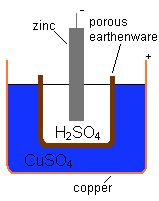
Schematic representation of Daniell's
original cell
A British chemist named
John Frederic Daniell searched for a way to eliminate the hydrogen
bubble problem found in the Voltaic Pile, and his solution was to use a
second electrolyte to consume the hydrogen produced by the first. In
1836, he invented the
Daniell cell, which consisted of a copper pot filled with a
copper sulphate solution, in which was immersed an unglazed
earthenware container filled with
sulphuric acid and a zinc electrode. The
earthenware barrier was porous, which allowed ions to pass through but
kept the solutions from mixing. Without this barrier, when no current
was drawn the copper ions would drift to the zinc anode and undergo
reduction without producing a current, which would destroy the battery's
life.
Over time, copper buildup would block the pores in the earthenware
barrier and cut short the battery's life. Nevertheless, the Daniel cell
provided a longer and more reliable current than the Voltaic cell
because the electrolyte deposited copper (a conductor) rather than
hydrogen (an insulator) on the cathode. It was also safer and less
corrosive. It had an operating voltage of roughly 1.1 volts. It saw
widespread use in telegraph networks until it was supplanted by the
Leclanché cell in the late 1860s.
1839 - The Grove
cell
The
Grove cell was invented by
William Robert Grove in 1839. It consisted of a zinc anode
dipped in
sulfuric acid
 and a
platinum cathode
dipped in
nitric acid, separated by porous
earthenware. The Grove cell provided a high current and nearly twice
the voltage of the Daniell cell, which made it the favored cell of the
American telegraph networks for a time. However, it gave off poisonous
nitric oxide fumes when operated.
The voltage also dropped sharply as the charge diminished, which became
a liability as telegraph networks grew more complex.
Platinum was also very expensive. The Grove cell was replaced by the
cheaper, safer and better performing gravity cell in the 1860s.
and a
platinum cathode
dipped in
nitric acid, separated by porous
earthenware. The Grove cell provided a high current and nearly twice
the voltage of the Daniell cell, which made it the favored cell of the
American telegraph networks for a time. However, it gave off poisonous
nitric oxide fumes when operated.
The voltage also dropped sharply as the charge diminished, which became
a liability as telegraph networks grew more complex.
Platinum was also very expensive. The Grove cell was replaced by the
cheaper, safer and better performing gravity cell in the 1860s.
1859 - The lead-acid cell: the first rechargeable battery
Up to this point, all existing batteries would be permanently drained
when all their chemical reactions were spent. In 1859,
Gaston Planté invented the
lead-acid battery, the first ever battery that could be recharged by
passing a reverse current through it. A lead acid cell consists of a
lead anode and a
lead oxide cathode immersed in
sulphuric acid. Both electrodes react with the acid to produce lead
sulfate, but the reaction at the lead anode releases electrons whilst
the reaction at the
lead oxide consumes them, thus producing a current. These chemical
reactions can be reversed by passing a reverse current through the
battery, thereby recharging it.
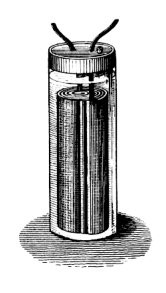
19th-century illustration of Planté's original lead-acid cell.
Planté's first model consisted of two lead sheets separated by rubber
strips and rolled into a spiral.
His batteries were first used to power the lights in train carriages
while stopped at a station. In 1881,
Camille Alphonse Faure invented an improved version that consisted
of a lead grid lattice into which a lead oxide paste was pressed,
forming a plate. Multiple plates could be stacked for greater
performance. This design was easier to mass-produce.
Compared to other batteries, Planté's was rather heavy and bulky for
the amount of energy it could hold. However, it could produce remarkably
large currents in surges. It also had very low internal resistance,
meaning a single battery could be used to power multiple circuits.
The lead-acid battery is still used today in automobiles and other
applications where weight isn't a big factor. The basic principle has
not changed since 1859, though in the 1970s a variant was developed that
used a gel electrolyte instead of a liquid (commonly known as a "gel
cell"), allowing the battery to be used in different positions
without failure or leakage.
Today cells are classified as "primary" if they produce a current
only until their chemical reactants are exhausted, and "secondary" if
the chemical reactions can be reversed by recharging the cell. The
lead-acid cell was the first "secondary" cell.
1860s - The
gravity cell
In the 1860s, a Frenchman by the name of Callaud invented a variant
of the
Daniell cell called the
gravity cell.
This simpler version dispensed with the porous barrier. This reduced the
internal resistance of the system and thus the battery yielded a
stronger current. It quickly became the battery of choice for the
American and British telegraph networks, and was used right up until the
1950s.
In the telegraph industry, this battery was often assembled on site by
the telegraph workers themselves, and when it ran down it could be
renewed by replacing the consumed components.
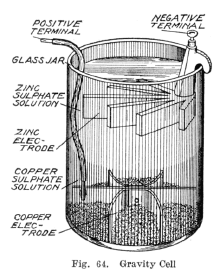
A 1919 illustration of a
gravity cell. This particular variant is also known as a
crowfoot cell due to distinctive shape of the electrodes.
The gravity cell consisted of a glass jar, in which a copper cathode
sat on the bottom and a zinc anode was suspended beneath the rim. Copper
sulfate crystals would be scattered around the cathode and then the jar
would be filled with distilled water. As the current was drawn, a layer
of zinc sulfate solution would form at the top around the anode. This
top layer was kept separate from the bottom copper sulfate layer by its
lower density and by the polarity of the cell.
The zinc sulfate layer was clear in contrast to the deep blue copper
sulfate layer, which allowed a technician to measure the battery life
with a glance. On the other hand, this setup meant the battery could
only be used in a stationary appliance, else the solutions would mix or
spill. Another disadvantage was that a current had to be continually
drawn to keep the two solutions from mixing by diffusion, so it was
unsuitable for intermittent use.
1866 - The
Leclanché cell
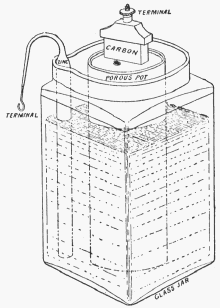
A 1912 illustration of a Leclanché cell.
In 1866,
Georges Leclanché invented
a battery that consisted of a zinc anode and a
manganese dioxide cathode wrapped in a porous material, dipped in a
jar of
ammonium chloride solution. The manganese dioxide cathode had a
little carbon mixed into it as well, which improved electrolyte
conductivity and absorption.
It provided a voltage of 1.4 to 1.6 volts.
This cell achieved very quick success in telegraphy, signaling and
electric bell work. It was used to power early telephones—usually from
an adjacent wooden box affixed to the wall—before telephones could draw
power from the line itself. It couldn't provide a sustained current for
very long. In lengthy conversations, the battery would run down,
rendering the conversation inaudible.
This was because certain chemical reactions in the cell increased the
internal resistance and thus lowered the voltage. These reactions
reversed themselves when the battery was left idle, so it was only good
for intermittent use.
1887 - The zinc-carbon cell: the first dry cell
In 1887 Carl Gassner patented a variant of the Leclanché cell which
came to be known as the
dry cell because it did not have a free liquid electrolyte. Instead,
the ammonium chloride was mixed with
Plaster of Paris to create a paste, with a bit of
zinc chloride added in to extend the shelf life. The
manganese dioxide cathode was dipped in this paste, and both were
sealed in a zinc shell which also acted as the anode.
Unlike previous wet cells, Gassner's dry cell was more solid, did not
require maintenance, did not spill and could be used in any orientation.
It provided a potential of 1.5 volts. The first mass-produced model was
the Columbia dry cell, first marketed by the National Carbon Company in
1896. The NCC improved Gassner's model by replacing the plaster of Paris
with coiled cardboard, an innovation which left more space for the
cathode and made the battery easier to assemble. It was the first
convenient battery for the masses and made portable electrical devices
practical. The
flashlight was invented that same year.
The
zinc-carbon battery (as it came to be known) is still manufactured
today.
In parallel, in 1887
Frederik Louis Wilhelm Hellesen developed his own dry cell design.
It has been claimed that Hellesen's design preceded that of Gassner.
1899 -
The nickel-cadmium battery
In 1899, a Swedish scientist named
Waldmar Jungner invented the
nickel-cadmium battery, a rechargeable battery that had nickel and
cadmium electrodes in a
potassium hydroxide solution. It was commercialized in Sweden in
1910 and reached the United States in 1946. The first models were robust
and had significantly better energy density than lead-acid batteries,
but were much more expensive.
1903 - The
nickel-iron battery
Jungner also invented a
nickel-iron battery the same year as his Ni-Cad battery, but found
it to be inferior to its cadmium counterpart and consequently never
bothered patenting it. It produced a lot more hydrogen gas when being
charged, meaning it couldn't be sealed, and the charging process was
less efficient (it was, however, cheaper). However,
Thomas Edison picked up Jugner's nickel-iron battery design,
patented it himself and sold it in 1903. Edison wanted to commercialise
a more lightweight and durable substitute for the lead-acid battery that
powered some early automobiles, and hoped that by doing so electric cars
would become the standard, with his firm as its main battery vendor.
However, customers found his first model to be prone to leakage and
short battery life, and it did not outperform the lead-acid cell by much
either. Although Edison was able to produce a more reliable and powerful
model seven years later, by this time the inexpensive and reliable
Model T Ford had made gasoline engine cars the standard.
Nevertheless, Edison's battery achieved great success in other
applications.
1955 -
The common alkaline battery
Up until the late 1950s the
zinc-carbon battery continued to be a popular primary cell battery,
but its relatively low battery life hampered sales. In 1955, an engineer
working for Eveready (now known as
Energizer) named
Lewis Urry was tasked with finding a way to extend the life of
zinc-carbon batteries, but Urry decided instead that alkaline batteries
held more promise. Up until then, longer-lasting alkaline batteries were
unfeasibly expensive. Urry's battery consisted of a manganese dioxide
cathode and a powdered zinc anode with an alkaline electrolyte. Using
powdered zinc gave the anode a greater surface area. These batteries hit
the market in 1959.
Early 1970s - The nickel hydrogen battery
The
nickel hydrogen battery entered the market as an energy-storage
subsystem for commercial communication satellites.
Late 1980s - The nickel metal-hydride battery
The first consumer grade
NiMH batteries for smaller applications
appeared on the market in 1989 as a variation of the 1970s
nickel hydrogen battery.
NiMH batteries tend to have longer lifespans than NiCd batteries (and
their lifespans continue to increase as manufacturers experiment with
new alloys) and, since cadmium
is toxic, NiMH batteries are less damaging to the environment.
1970s and 1990s - The lithium and lithium-ion batteries
Lithium is the metal with lowest density and has the greatest
electrochemical potential and energy-to-weight ratio, so in theory it
would be an ideal material with which to make batteries. Experimentation
with
lithium batteries began in 1912 under G.N. Lewis, and in the 1970s
the first lithium batteries were sold.
In the 1980s, an American chemist
John B. Goodenough led a research team at Sony that would produce
the
lithium ion battery, a rechargeable and more stable version of the
lithium battery; the first ones were sold in 1991.
In 1996, the
lithium ion polymer battery was released. These batteries hold their
electrolyte in a solid polymer composite instead of a liquid solvent,
and the electrodes and separators are laminated to each other. The
latter difference allows the battery to be encased in a flexible
wrapping instead of a rigid metal casing, which means such batteries can
be specifically shaped to fit a particular device. They also have a
higher energy density than normal lithium ion batteries. These
advantages have made it a choice battery for portable electronics such
as mobile phones and
PDAs, as they allow for more flexible and compact design.
